沙特SFDA---医疗器械/IVD概述
在2019-2022年期间,沙特食品药品监督管理局 (SFDA) 更新了沙特阿拉伯的医疗器械法规,增加了SFDA注册的复杂性。特别是从2022年一月一日开始,参考GHTF计划正式取消,只能采用TFA注册模式;这影响了医疗器械的分类,改变了医疗器械上市许可MDMA(高危申请)的概念和内容。此外,我们预计 MDNR(医疗器械国家登记处)将在 2022 年消失,这是著名的低风险器械程序。因此,沙特阿拉伯的授权代表 (AR)面临着重大项目,因为所有先前批准的设备、IVD 和简单的医疗用品都必须符合新的法规。与欧盟 MDR 类似,申请MDMA需要更多文件、测试报告和获得批准的时间。
沙特的医疗器械法规变化
曾经,根据沙特阿拉伯的医疗器械暂行规定,SFDA 认可欧盟、美国、加拿大、澳大利亚和日本等其他 GHTF 国家的特定批准,简化了沙特阿拉伯的授权流程。
目前,SFDA 于 2021 年底取消了 GHTF 路线。 从 2022 年 1 月开始,所有 MDMA 申请(高风险器械)都必须经过技术文件评估(TFA)程序。最近几个月,SFDA 修订了许多受 MDR 和 IVDR 影响的要求。因此,MDMA 申请的注册要求变得非常严格,并出现了更多条件,例如更新的临床评估报告 CER 以及需要在沙特阿拉伯进行上市后临床随访 PMCF 研究。另一方面,从2022年9月起,低风险医疗器械的上市程序(注册)将不再可用。所有低风险医疗器械将需要通过MDMA途径重新注册,这需要TFA申请。
医疗器械产品分类
通常,SFDA 医疗器械分类将遵循参考国家的相同类别。SFDA 参考欧盟MDR和IVDR分类。但是,也有一些特殊案例,比如,产品在其本国被视为低风险医疗器械,但在沙特被视为非医疗器械,反之亦然。SFDA 医疗器械分类为 A、B、C 或 D 类。这是根据其风险等级而定。分类是确定注册程序及其要求所必需的,如下一部分所述。
SFDA MDS-G5 文件详细说明了医疗器械分类规则(类似于欧洲 MDR 分类)下图 。
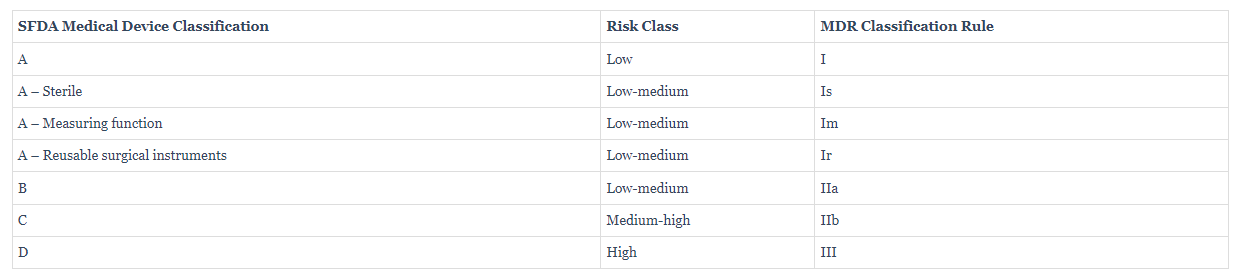
在 SFDA (MDS-G42) 指南中,我们可以找到对 SFDA 分类规则的更多说明。
在体外诊断方面,SFDA 也在采用欧盟医疗器械法规 IVDR:

沙特SFDA---医疗器械IVD注册
沙特阿拉伯的医疗器械公司必须根据沙特阿拉伯公布的医疗器械暂行规定在 SFDA 注册其产品并获得医疗器械营销授权 MDMA 证书。高风险设备需要 MDMA,而低风险(非无菌/非测量)不需要长期的MDMA,但它们在 SFDA 有其上市程序。而且低风险设备(非测量/非无菌)不需要授权代表。
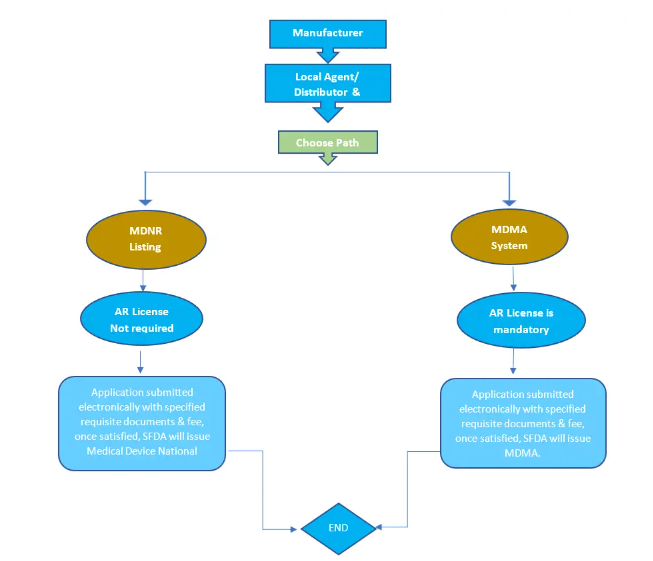
1- 低风险(I类)医疗器械注册
SFDA 要求低风险医疗器械(医疗用品)公司在医疗器械国家注册局 MDNR 中注册其产品。这是一个仅适用于低风险设备、I 类但非无菌和非测量产品的列名程序。
有两种低风险注册途径(非无菌、非测量)。路径及其要求如下:
1.1精简路线要求(参考GHTF国家)
· 产品标签
· 使用说明 (IFU)
· ISO 13485:2016
· 国家(沙特阿拉伯)特定的符合性声明
在参考司法管辖区注册的证据

1.2直接路线要求
· 产品标签
· 使用说明 (IFU)
· ISO 13485:2016
· 国家(沙特阿拉伯)特定的符合性声明
请注意,上述文件是注册 I 类(低风险、非无菌、非测量设备)的标准要求。但是,值得一提的是,SFDA 审查员有可能会要求提供额外的要求或信息。
1.3 注册有效期 三年
1.4 申请周期
沙特阿拉伯的低风险医疗器械注册时限通常为1至2周;这取决于 SFDA 询问的数量和公司需要回复的时间。
1.5 费用
I 类低风险医疗器械SFDA费用:SAR 500 / 器械
1.6 续证
可在医疗器械有效期届满前 (60) 天前进行注册续证
2-高风险医疗器械注册(MDMA)
正如我们前面提到的,沙特阿拉伯的高风险医疗器械需要任命一名沙特阿拉伯授权代表 AR并在医疗器械营销授权 (MDMA) 途径中进行注册。在提交产品申请之前,必须执行 AR 协议和随后的 SFDA ARL许可证。
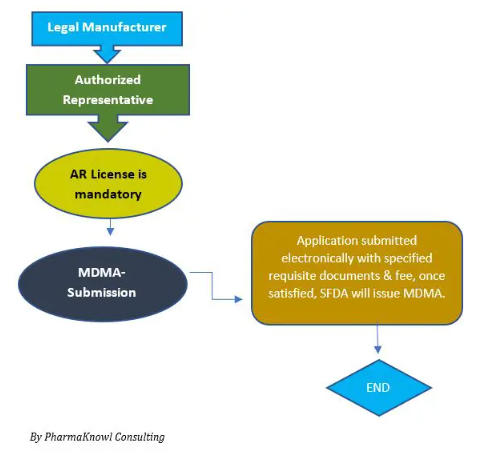
2.1 MDMA/TFA技术文件——注册要求
· 申请表中的内容
· 详细的设备描述/预期用途/设备历史/分类。
· 设备标签和使用说明
· 设计和制造信息
· 安全与性能基本原则(以前称为基本要求清单
· 收益风险分析
· 风险管理文件(计划和报告)
· 产品验证和确认,包括
· 临床前测试和测试报告,包括生物相容性测试报告。
· 临床研究计划和报告
· 临床评估报告 CER
· 上市后临床随访 (PMCF)
· 上市后监督 (PMS)、计划和报告
· 定期安全更新报告 (PSUR) – 适用于 B、C 和 D 类设备
除上述文件外,SFDA 官员可能会在申请过程中要求提供更多信息。
2.2 注册有效期 三年
2.3 申请费用和周期

2.4 其他注意事项
· 申请人必须在 30 天内支付 SFDA 费用。
· 当SFDA因在(60)天内未回复而退回申请时,提交的申请将被删除且不予退款。
· 如果申请人在三个试验期内未作出答复,SFDA 将拒绝提交的申请,不退还费用。
· 医疗器械公司可以在当前 MDMA 证书到期前两个月(60 天)开始申请续签。
· 医疗器械公司可以随时在现有文档中启动应用程序更新。
· 总部位于沙特的公司必须获得 SFDA 医疗器械企业许可证 MDEL 才能提交注册申请或进行医疗器械的分销。
沙特SFDA---授权代表AR
在沙特阿拉伯,沙特食品和药物管理局 (SFDA) 要求医疗器械制造商指定授权代表 (AR) 公司在任何时候代表他们在市场上行事。AR 负责 医疗器械注册提交、符合 SFDA 法规、安全性以及所需监管标准的实施。过期的 AR 会影响港口的货物清关,因此最好密切关注其有效性。需要时,可以在没有先前指定的 AR 参与的情况下更改授权代表。
哪些公司可以做AR?
由于授权代表必须是沙特当地公司,合法制造商有两种选择。第一个是指定他们的当地经销商作为授权代表,第二个选项是指定一个第三方公司作为授权代表。在大多数情况下,海外制造商会选择沙特当地的第三方公司作为其在沙特的AR.
什么是AR授权代表?
一家沙特公司与一家医疗器械合法制造商签署协议,代表其在沙特阿拉伯行事。授权代表需经国家食品药品监督管理局注册批准,并不一定拥有市场上的任何商业权利;它通过确保合规性来促进营销和销售过程。
所有医疗设备都需要 AR 吗?
只有高风险的医疗器械才需要。因此,低风险 1 (A) 类设备不需要在沙特阿拉伯指定当地授权代表。

AR的责任
· 与SFDA联系的代表处
· 医疗器械上市许可提交及维护
· 列出其他医疗器械
· 落实国家食品药品监督管理局要求的行动
· 向 SFDA 提供与质量、有效性和安全性相关的数据。
· 上市后监督
· 提交在国外发生的不利行为
· 提交由持续监督产生的任何必要的纠正措施
· 与负责医疗器械安装、维护和供应的其他方的参与
AR 许可要求
· 当地沙特公司
· 建立国家登记号码 (MDNR)
· 有效的 SFDA 医疗器械活动设立许可证 (MDEL)
· 质量管理体系(QMS)
· 与合法制造商持有授权代表协议
SFDA授权代表协议
SFDA 要求为医疗器械授权代表签署具体的协议模板。合法制造商和沙特当地公司可以按原样签署,因为它具有最低要求的条款。
注意:
· 授权代表应对其在 KSA 内代表的每个制造商签订单独的协议。
· 制造商可以为每个医疗器械类别或组指定不同的 AR,因此可以达成不同的协议。
· AR 协议一经签署,必须由双方在各自国家/地区进行合法化(大使馆公证)。
AR 协议的有效期至少为一年。请注意,签发的 AR 许可证的有效期不能长于 AR 协议的有效期。申请人可以提交有效期为一年、两年等的AR申请,并收取相应的费用。
AR的更改
无需先前指定的 AR 参与即可更改在沙特的授权代表。这是一个需要 2 周的过程,然后是相关医疗器械营销授权 (MDMA) 许可证的转让。
ARL许可费
授权代表证书的 SFDA 许可费为 2,600 沙特里亚尔/年
ARL时间 4-6周
SFDA网站:https://www.sfda.gov.sa/ar/overview
联系人:Jacky cheng 13958111261( 同微信 ) , Email:dowell001@cccdw.cn


-
ECE R128 欧洲车用LED灯泡E/mark标......
2024-05-08
-
2024-05-08
-
2024-05-08
-
2024-04-08
-
2024-03-14
-
2024-03-14
-
2022-09-16
-
新加坡HSA---未注册的医疗器械如何参加新加坡展......
2022-09-16
-
2022-09-07
-
2022-09-03
-
2022-01-13
-
2022-01-07